Score- Survey on Clindamycin and Benzoyl Peroxide Outcome in Reducing Acne by Experts
Abstract
Background: Topical combination therapies are the cornerstone of acne vulgaris treatment, with clindamycin-benzoyl peroxide combinations widely prescribed for synergistic antimicrobial effects. Limited data exists on dermatology practitioners' real-world experiences and prescribing patterns with these formulations across diverse Indian populations.
Objective: To evaluate dermatology practitioners' perspectives on clindamycin-benzoyl peroxide (Clin-B) combination therapy effectiveness, safety satisfaction, prescribing patterns across acne severity levels, and antibiotic stewardship implementation.
Methods: A cross-sectional survey using the SCORE (Survey on Clinred-B's Outcome in Reducing Acne by Experts) questionnaire was conducted among dermatology practitioners across six Indian states (Maharashtra, Rajasthan, Uttar Pradesh, Gujarat, Madhya Pradesh, and Telangana). The 20-question survey assessed treatment utilization, safety satisfaction, comparative effectiveness, clinical decision-making factors, and stewardship awareness. Data were analyzed using descriptive statistics.
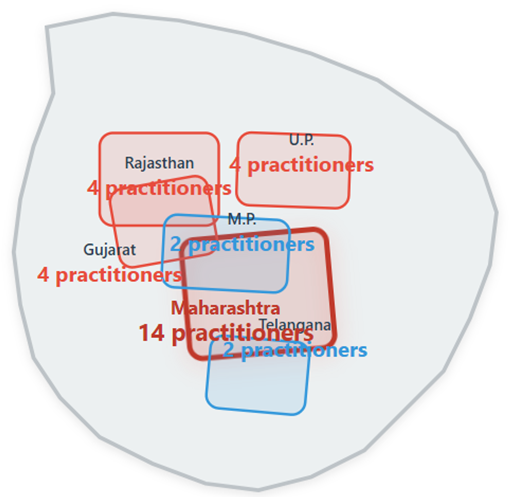
Results: Thirty dermatology practitioners participated, with the largest representation from Maharashtra (46.7%). High combination therapy adoption was observed (66.7% using in >50% of patients). Safety satisfaction was universal (100% satisfied; 80% very satisfied). Treatment preferences demonstrated clear severity stratification: mild acne 40%, moderate 50%, moderately severe 56.7%, and severe acne 70% preferred Clin-B. Perfect antibiotic stewardship awareness was evident (100% agreement on avoiding monotherapy). Practitioners preferred once-daily night-time dosing (66.7%) and rated Clin-B favorably in comparative effectiveness assessments (73.3% versus clindamycin-adapalene; 66.7% versus adapalene-benzoyl peroxide).
Conclusion: Dermatology practitioners demonstrate strong confidence in Clin-B therapy with evidence-based, severity-stratified prescribing (40-70% preference increase). Universal safety satisfaction and perfect stewardship compliance indicate successful resistance prevention implementation. Results provide real-world validation supporting current guidelines recommending combination therapy as first-line acne treatment.
Downloads
References
Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
Sardana K, Sharma RC, Sarkar R. Seasonal variation in acne vulgaris--myth or reality. J Dermatol. 2002;29(8):484-488.
Ghodsi SZ, Orawa H, Zouboulia E. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129(9):2136-2141.
Dréno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448-456.
Tan J, Tanghetti EA, Harper JC, et al. Current concepts in topical therapy for acne vulgaris. Dermatol Clin. 2016;34(2):159-172.
Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140(4):672-676.
Law MPM, Chuh AAT, Lee A, Molinari N. Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clin Exp Dermatol. 2010;35(1):16-21.
Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-50.
Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.e33.
Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261-1268.
Burkhart CG, Burkhart CN. Treatment of acne vulgaris without antibiotics: tertiary amine-benzoyl peroxide combination vs. benzoyl peroxide alone (Part I). Int J Dermatol. 2007;46(1):89-93.
Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e23-33.
Del Rosso JQ, Harper JC, Graber EM, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: Part 1. Antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance. J Clin Aesthet Dermatol. 2016;9(1):18-24.
Schafer F, Fich F, Lam M, et al. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52(4):418-425.
Groves RM, Fowler FJ Jr, Couper MP, et al. Survey Methodology. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2009.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457.
Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163-194.
Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20(1):54-60.
Sedgwick P. Cross sectional studies: advantages and disadvantages. BMJ. 2014;348:g2276.
Levin KA. Study design III: Cross-sectional studies. Evid Based Dent. 2006;7(1):24-25.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194.
Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701-2711.
Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 7th ed. New York: Oxford University Press; 2012.
Grady C. Payment of clinical research subjects. J Clin Invest. 2005;115(7):1681-1687.
Dickert N, Grady C. What's the price of a research subject? Approaches to payment for research participation. N Engl J Med. 1999;341(3):198-203.
Council for International Organizations of Medical Sciences. International Ethical Guidelines for Health-related Research Involving Humans. Geneva: CIOMS; 2016.
Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305-310.
Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312(7023):71-72.
Bartlett JE, Kotrlik JW, Higgins CC. Organizational research: determining appropriate sample size in survey research. Inf Technol Learn Perform J. 2001;19(1):43-50.
Krejcie RV, Morgan DW. Determining sample size for research activities. Educ Psychol Meas. 1970;30(3):607-610.
Cochran WG. Sampling Techniques. 3rd ed. New York: John Wiley & Sons; 1977.
Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. 7th ed. New York: John Wiley & Sons; 1999.
Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: Sage Publications; 2002.
Teddlie C, Yu F. Mixed methods sampling: a typology with examples. J Mixed Methods Res. 2007;1(1):77-100.
Marshall MN. Sampling for qualitative research. Fam Pract. 1996;13(6):522-525.
Hulley SB, Cummings SR, Browner WS, et al. Designing Clinical Research. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
Polit DF, Beck CT. Nursing Research: Generating and Assessing Evidence for Nursing Practice. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619-625.
Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635-641.
Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method. 4th ed. Hoboken, NJ: John Wiley & Sons; 2014.
Fowler FJ Jr. Survey Research Methods. 5th ed. Thousand Oaks, CA: Sage Publications; 2013.
Oppenheim AN. Questionnaire Design, Interviewing and Attitude Measurement. 2nd ed. London: Continuum; 1992.
Bradburn NM, Sudman S, Wansink B. Asking Questions: The Definitive Guide to Questionnaire Design. San Francisco: Jossey-Bass; 2004.
Krosnick JA, Presser S. Question and questionnaire design. In: Marsden PV, Wright JD, eds. Handbook of Survey Research. 2nd ed. Bingley: Emerald Group Publishing; 2010:263-314.
Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
Haynes SN, Richard DCS, Kubany ES. Content validity in psychological assessment: a functional approach to concepts and methods. Psychol Assess. 1995;7(3):238-247.
Polit DF, Beck CT, Owen SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health. 2007;30(4):459-467.
Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35(6):382-385.
Davis LL. Instrument review: Getting the most from a panel of experts. Appl Nurs Res. 1992;5(4):194-197.
Fowler FJ Jr, Mangione TW. Standardized Survey Interviewing: Minimizing Interviewer-Related Error. Newbury Park, CA: Sage Publications; 1990.
Groves RM, Magilavy LJ. Measuring and explaining interviewer effects in centralized telephone surveys. Public Opin Q. 1986;50(2):251-266.
Cannell CF, Miller PV, Oksenberg L. Research on interviewing techniques. Sociol Methodol. 1981;12:389-437.
Dillman DA. Mail and Internet Surveys: The Tailored Design Method. 2nd ed. New York: John Wiley & Sons; 2000.
Fowler FJ Jr. Improving Survey Questions: Design and Evaluation. Thousand Oaks, CA: Sage Publications; 1995.
Lessler JT, Kalsbeek WD. Nonsampling Error in Surveys. New York: John Wiley & Sons; 1992.
Van den Broeck J, Cunningham SA, Eeckels R, Herbst K. Data cleaning: detecting, diagnosing, and editing data abnormalities. PLoS Med. 2005;2(10):e267.
Maletic JI, Marcus A. Data cleansing: beyond integrity analysis. In: Proceedings of the Conference on Information Quality. Cambridge, MA: MIT; 2000:200-209.
Osborne JW. Best Practices in Data Cleaning: A Complete Guide to Everything You Need to Do Before and After Collecting Your Data. Thousand Oaks, CA: Sage Publications; 2013.
Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991.
Armitage P, Berry G, Matthews JN. Statistical Methods in Medical Research. 4th ed. Oxford: Blackwell Science; 2002.
Agresti A. Categorical Data Analysis. 3rd ed. Hoboken, NJ: John Wiley & Sons; 2012.
Agresti A. An Introduction to Categorical Data Analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2007.
Kish L. Survey Sampling. New York: John Wiley & Sons; 1965.
Lohr SL. Sampling: Design and Analysis. 2nd ed. Boston: Brooks/Cole; 2009.
Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.e33.
Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261-1268.
Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-50.
Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(1 Suppl):S1-37.
Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin conditions: focus on antibiotic resistance. Cutis. 2007;79(6 Suppl):9-25.
Harper JC, Thiboutot DM. Pathogenesis of acne: recent research advances. Adv Dermatol. 2003;19:1-10.
Gold MH, Biron JA, Wilson DM. Treatment of acne vulgaris with a combination clindamycin and benzoyl peroxide gel compared with benzoyl peroxide alone and vehicle gel: a multicentre, randomized, double-blind study. Br J Dermatol. 2009;160(6):1277-1281.
Langner A, Shear N, Bikowski J, et al. A randomized, double-blind, parallel group study comparing a clindamycin phosphate 1.2%/benzoyl peroxide 2.5% gel combination with its vehicle for the treatment of acne vulgaris. Cutis. 2007;80(6):482-489.
Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36(6):416-418.
Lucky AW, Barber BL, Girman CJ, et al. A multirater validation study to assess the reliability of acne lesion counting. J Am Acad Dermatol. 1996;35(4):559-565.
Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e23-33.
Del Rosso JQ, Harper JC, Graber EM, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: Part 1. Antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance. J Clin Aesthet Dermatol. 2016;9(1):18-24.
Burkhart CG, Burkhart CN. Treatment of acne vulgaris without antibiotics: tertiary amine-benzoyl peroxide combination vs. benzoyl peroxide alone (Part I). Int J Dermatol. 2007;46(1):89-93.
Kawashima M, Nagare T, Doi M. Clinical effectiveness and safety of benzoyl peroxide for acne vulgaris: comparison between Japanese and Western patients. J Dermatol. 2017;44(11):1212-1218.
Cunliffe WJ, Holland KT, Bojar R, Levy SF. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24(7):1117-1133.
Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37(4):590-595.
Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361-372.
Tan J, Tanghetti EA, Harper JC, et al. Current concepts in topical therapy for acne vulgaris. Dermatol Clin. 2016;34(2):159-172.
Yentzer BA, Hick J, Reese EL, et al. Adherence to topical therapy increases around the time of office visits. J Am Acad Dermatol. 2009;60(1):81-87.
Dréno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448-456.
Groves RM, Fowler FJ Jr, Couper MP, et al. Survey Methodology. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2009.
Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
Barbieri JS, Spaccarelli N, Margolis DJ, James WD. Assessment of the incidence of drug-resistant Propionibacterium acnes in patients with acne vulgaris. JAMA Dermatol. 2017;153(4):323-326.
Ross JI, Snelling AM, Carnegie E, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148(3):467-478.
Eichenfield LF, Krakowski AC, Piggott C, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131 Suppl 3:S163-186.
Simonart T. Newer approaches to the treatment of acne vulgaris. Am J Clin Dermatol. 2012;13(6):357-364.
Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73(8):779-787.
Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: Part I. Pregnancy. J Am Acad Dermatol. 2014;70(3):401.e1-14.
Moore A, Green LJ, Bruce S, et al. Once-daily oral lymecycline versus twice-daily topical clindamycin in the treatment of mild to moderate acne vulgaris. Clin Exp Dermatol. 2001;26(7):609-615.
Alexis AF, Burgess C, Dawson CT, et al. Multicenter randomized controlled trial of a topical suspension of encapsulated benzoyl peroxide in adult acne. J Drugs Dermatol. 2016;15(10):1235-1242.
Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(1 Suppl):S1-37.
Ramos-e-Silva M, Carneiro SC. Acne vulgaris: review and guidelines. Dermatol Nurs. 2009;21(2):63-68.
Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140(4):672-676.
Jankovic S, Vukicevic J, Djordjevic S, et al. Quality of life among school children with acne: results of a cross-sectional study. Indian J Dermatol Venereol Leprol. 2012;78(4):454-458.
Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-77.
Goodman G. Cleansing and moisturizing in acne patients. Am J Clin Dermatol. 2009;10 Suppl 1:1-6.
Pariser DM, Rich P, Cook-Bolden FE, Korotzer A. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13(9):1083-1089.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457.
Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20(1):54-60.
Bartlett JE, Kotrlik JW, Higgins CC. Organizational research: determining appropriate sample size in survey research. Inf Technol Learn Perform J. 2001;19(1):43-50.
Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. 7th ed. New York: John Wiley & Sons; 1999.
Levin KA. Study design III: Cross-sectional studies. Evid Based Dent. 2006;7(1):24-25.
Sedgwick P. Cross sectional studies: advantages and disadvantages. BMJ. 2014;348:g2276.
Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619-625.
Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635-641.
Groves RM, Fowler FJ Jr, Couper MP, et al. Survey Methodology. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2009.
Hulley SB, Cummings SR, Browner WS, et al. Designing Clinical Research. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
Tourangeau R, Rips LJ, Rasinski K. The Psychology of Survey Response. Cambridge: Cambridge University Press; 2000.
Sudman S, Bradburn NM, Schwarz N. Thinking About Answers: The Application of Cognitive Processes to Survey Methodology. San Francisco: Jossey-Bass; 1996.
Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;76(6):1068-1076.e9.
Rivera R, Guerra A. Management of acne in women over 25 years of age. Actas Dermosifiliogr. 2009;100(1):33-37.
Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56-59.
Law MPM, Chuh AAT, Lee A, Molinari N. Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clin Exp Dermatol. 2010;35(1):16-21.
Tanghetti EA, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11(12):1417-1421.
Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153(2):395-403.
Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425.
Ghodsi SZ, Orawa H, Zouboulia E. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129(9):2136-2141.
Sardana K, Sharma RC, Sarkar R. Seasonal variation in acne vulgaris--myth or reality. J Dermatol. 2002;29(8):484-488.
Clinical Excellence Commission. Antimicrobial stewardship clinical care standard. Sydney: CEC; 2017.
Public Health England. Management and treatment of common infections: antibiotic guidance for primary care. London: PHE; 2018.
Eady EA, Gloor M, Leyden JJ. Propionibacterium acnes resistance: a worldwide problem. Dermatology. 2003;206(1):54-56.
Schafer F, Fich F, Lam M, et al. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52(4):418-425.