Assessment of knowledge, attitude, and practice of pharmacovigilance in a medical college of Nepal
Abstract
Introduction: Pharmacovigilance is the science that relates to the collection, detection, assessment, monitoring, and prevention of adverse drug reactions (ADR). The incidence of ADR is 2.4-6.5% in western countries, with only 6-10% reported worldwide. The under-reporting of ADR is due to inadequate knowledge, attitude, and practice among the prescribers about the system. Thus, medical students who are the future drug prescribers bear a crucial role in bridging this gap.
Materials and Methods: This cross-sectional questionnaire survey was carried out among 261 medical undergraduates including interns at Maharajgunj Medical Campus in Kathmandu. A self-administered questionnaire was used for data collection. The responses were analyzed and descriptive statistics are presented as frequencies and percentages.
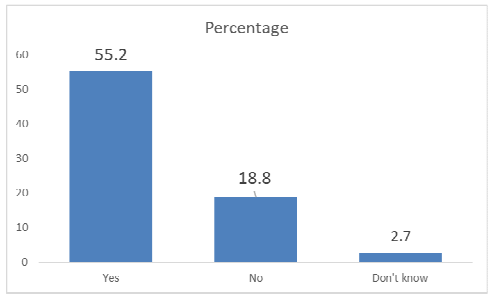
Results: In this study, 94.3% of students responded that doctors, nurses, and pharmacists need to report ADR as they encounter them but only 13.8% responded to have reported ADR to the concerned authority. Also, 97.3% of respondents answered that ADR reporting should be taught in their medical college.
Conclusion: In conclusion, medical students are not adequately aware of the ADR reporting system. A proper orientation to pharmacovigilance should be given to the medical students by incorporating it into the medical curriculum and providing training to future healthcare professionals.
Downloads
References
World Health Organization (WHO). The importance of pharmacovigilance: Safety monitoring of medicinal products [internet]. Geneva: WHO; 2002. Available from: https://apps.who.int/iris/bitstream/handle/10665/42493/a75646.pdf [Full Text]
Palaian S, Ibrahim MI, Mishra P. Health professionals’ knowledge, attitude and practices towards pharmacovigilance in Nepal. Pharm Pract (Granada). 2011;9:228-235. [PubMed | Full Text | DOI]
Dhananjay K, Himasri E. A study of assessing knowledge, attitude and practice of pharmacovigilance among medical students of a South Indian teaching hospital. Int J Basic Clin Pharmacol. 2017; 6:43-47. [Full Text | DOI]
Sharma R, Kellarai A. Pharmacovigilance and adverse drug reaction reporting perspectives among interns and postgraduates of a teaching hospital. J Pharmacol Pharmacother [Internet]. 2014; 5(4):248-250. [PubMed | Full Text | DOI]
Alemu BK, Biru TT. Health care professionals’ knowledge, attitude, and practice towards adverse drug reaction reporting and associated factors at selected public hospitals in northeast Ethiopia: A cross-sectional study. Biomed Res Int. 2019; 8690546:11. [Full Text | DOI]
Patel PB, Patel TK, Anturlikar S, Khatun S, Bhabhor P, Saurabh MK. Adverse drug reactions reporting by undergraduate medical students in a tertiary care teaching hospital of India: Content and quality analysis in comparison to physician reporting. Perspect Clin Res. 2017;8:137-144. [PubMed | Full Text | DOI]
Desai CK, Iyer G, Panchal J, Shah S, Dikshit RK. An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting among prescribers at a tertiary care hospital. Perspect Clin Res. 2011; 2:129-136. [PubMed | Full Text | DOI]
Kamtane RA, Jayawardhani V. Knowledge, attitude, and perception of physicians towards adverse drug reaction (ADR) reporting: A pharmacoepidemiological study. Asian J Pharm Clin Res. 2012; 5:210-214. [Full Text]
Parthiban G, Nileshraj G, Mangaiarkkarasi A, Meher AR. A Survey on Knowledge, Attitude, and Awareness of Pharmacovigilance among Medical Students in a Teaching Hosptial, Puducherry. Indian J Basic Appl Med Res. 2015; 5:198-203. [Full Text]
Upadhyaya HB, Vora MB, Nagar JG, Patel PB. Knowledge, attitude, and practices toward pharmacovigilance and adverse drug reactions in postgraduate students of Tertiary Care Hospital in Gujarat. J Adv Pharm Technol Res. 2015;6:29-34.[PubMed | Full Text | DOI]
Hema NG, Bhuvana KB, Sangeetha. Pharmacovigilance: The extent of awareness among the final year students, interns and postgraduates in a government teaching hospital. J Clin Diagn Res. 2019; 6:1248-1253. [Full Text]
Agarwal R, Daher AM, Mohd Ismail N. Knowledge, practices and attitudes towards adverse drug reaction reporting by private practitioners from klang valley in malaysia. Malays J Med Sci. 2013;20:52-61. [ PubMed | Full Text]
Ganesan S, Vikneswaran G, Reddy KC, Subrahmanyam DK, Adithan C. A survey on knowledge, attitude and practice of pharmacovigilance towards adverse drug reactions reporting among doctors and nurses in a tertiary care hospital in South India. J Young Pharm. 2016;8:471-476.[Full Text | DOI]
Gupta SK, Nayak RP, Shivaranjani R, Vidyarthi SK. A questionnaire study on the knowledge, attitude, and practice of pharmacovigilance among healthcare professionals in a teaching hospital in South India. Perspect Clin Res. 2015;6:45-52. [PubMed | Full Text | DOI]
Avery AJ, Anderson C, Bond CM, Fortnum H, Gifford A, Hannaford PC, et al. Evaluation of patient reporting of adverse drug reactions to the UK “Yellow Card Scheme”: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess. 2011; 15:1-234, iii–iv. [PubMed | Full Text | DOI]
Kharkar M, Bowalekar S. Knowledge, attitude and perception/practices (KAP) of medical practitioners in India towards adverse drug reaction (ADR) reporting. Perspect Clin Res. 2012; 3:90-94. [PubMed |Full Text | DOI]
Gavaza P, Brown CM, Khoza S. Texas pharmacists’ opinions on reporting serious adverse drug events to the Food and Drug Administration: a qualitative study. Pharm World Sci. 2010; 32:651-657. [PubMed| DOI]
Nichols V, Theriault-Dube I, Touzin J, Delisle JF, Lebel D, Bussieres JF, et al. Risk perception and reasons for noncompliance in pharmacovigilance: aqualitative study conducted in Canada. Drug Saf. 2009;32:579-590. [PubMed |DOI]