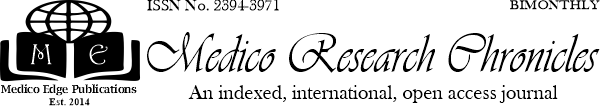Efficacy and safety of rivaroxaban in the treatment of deep vein thrombosis.
Abstract
Objectives: This study aims to present the clinical results of patients administered rivaroxaban for the treatment of deep vein thrombosis (DVT) in terms of efficacy and safety. \
Methods: A total of 50 patients (30 males, 20 females age range 25 to 88 years) diagnosed with DVT had received rivaroxaban treatment in the department of medicine, Chhattisgarh Institute of Medical Science (CIMS) Bilaspur Chhattisgarh India. between September 2018 and august 2020 were included in the study. Deep vein thrombosis diagnoses of the patients were confirmed by Doppler ultrasonography. The patients’ epidemiological and biochemical values were evaluated. Major- minor bleeding and recurrence that occurred during rivaroxaban treatment was investigated.
Results: Patients were treated for 3, 6, or 12, months, according to flow up and result. When anticoagulant treatments of the patients were examined, 15 patients (30%) were treated with rivaroxaban as initial treatment and 35 patients (70%) had transitioned from warfarin to rivaroxaban treatment. In patients using rivaroxaban, one patient had hypermenorrhea and two patients had epistaxis. Major bleeding was not detected. While three patients had alanine aminotransferase levels up to two times higher than the normal limit, none of the patients had clinically significant liver or kidney failure. Recurrent DVT or pulmonary embolism was not detected in the patients.
Conclusion: According to the current guidelines and literature findings novel oral anticoagulants could be used safely and efficiently as first-line drug therapy in DVT patients due to their non-inferior effectiveness to warfarin and lower side effect profile.
Downloads
References
Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59- 7939-an oral, direct Factor Xa inhibitor. J Thromb Haemost 2005;3:514-21.
Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939--an oral, direct Factor Xa inhibitor--after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005;61:873-80.
Bansilal S, Bloomgarden Z, Halperin JL, Hellkamp AS, Lokhnygina Y, Patel MR, et al. Efficacy and safety of rivaroxaban in patients with diabetes and nonvalvular atrial fibrillation: the Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF Trial). Am Heart J 2015;170:675-82
Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510.
Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97
Beyan C. The Traditional and new oral anticoagulants. Turkiye Klinikleri J Hematol-Special Topics 2016;9:68-75
. Witt DM, Clark NP, Kaatz S, Schnurr T, Ansell JE. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis 2016;41:187-205.
Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis 2013;35:312-9
Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003;139:893-900.
Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004;141:745-52.
Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy: recent data and ideas. Stroke 2005;36:1588-93
Altay S, Yıldırımtürk Ö, Çakmak HA, Aşkın L, Sinan ÜY, Beşli F, et al. New oral anticoagulants-TURKey (NOAC-TURK): Multicenter cross-sectional study. Anatol J Cardiol 2017;17:353-61
Herman D, Peternel P, Stegnar M, Breskvar K, Dolzan V. The influence of sequence variations in factor VII, gammaglutamyl carboxylase and vitamin K epoxide reductase complex genes on warfarin dose requirement. Thromb Haemost 2006;95:782-7.
Coumadin® Tablets (Warfarin Sodium Tablets, USP) Crystalline Coumadin® For Injectıon (Warfarin Sodium for Injection, USP). Available from: https://www.accessdata.
Romualdi E, Donadini MP, Ageno W. Oral rivaroxaban after symptomatic venous thromboembolism: the continued treatment study (EINSTEIN-extension study). Expert Rev Cardiovasc Ther 2011;9:841-4
Yamada N, Hirayama A, Maeda H, Sakagami S, Shikata H, Prins MH, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism - the J-EINSTEIN DVT and PE program. Thromb J 2015;13:2
Castellucci LA, de Wit K, Garcia D, Ortel TL, Le Gal G. Extended anticoagulation for unprovoked venous thromboembolism. Res Pract Thromb Haemost 2018;2:529-34.
Desai J, Kolb JM, Weitz JI, Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants--defining the issues and the management strategies. Thromb Haemost 2013;110:205-12.
Highlights of prescribing information of rivaroxaban for FDA approval. Available from: https://www.accessdata. fda.gov/drugsatfda_docs/label/2016/202439s017lbl.pdf
Wattanakit K, Cushman M. Chronic kidney disease and venous thromboembolism: epidemiology and mechanisms. Curr Opin Pulm Med 2009;15:408-12.
Bonde AN, Lip GY, Kamper AL, Hansen PR, Lamberts M, Hommel K, et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014;64:2471-82.
Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost 2016;14:461-7.
Monahan RC, Suttorp MM, Gabreëls BATF. A case of rivaroxaban-associated acute tubulointerstitial nephritis. Neth J Med 2017;75:169-71
Oliveira M, Lima C, Góis M, Viana H, Carvalho F, Lemos S. Rivaroxaban-related nephropathy. Port J Nephrol Hypert 2017;31:212-6
Russmann S, Niedrig DF, Budmiger M, Schmidt C, Stieger B, Hürlimann S, et al. Rivaroxaban postmarketing risk of liver injury. J Hepatol 2014;61:293-300