Clinical and laboratory profile of patients with disorders of sex development: Experience from two tertiary care hospitals in Bangladesh
Abstract
Disorders of sex development (DSD) are relatively rare conditions where gender assignment remains uncertain, presented with ambiguous genitalia in newborns, and atypical sex development in adolescents. Management remains a challenge for all professionals involved and largely depends on the participatory factors responsible for the causation of the disorders.
Patients and Methods: All patients with the complaint of atypical features of sex development, attending the pediatric endocrine unit of two tertiary level hospitals in Dhaka city, in a period of 24 months from May 2017 to May 2019 were incorporated in this study and their clinical, hormonal and cytogenetic findings have been documented.
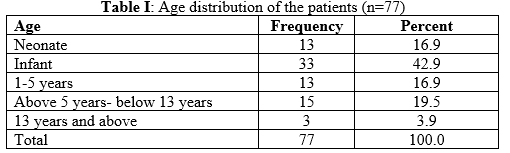
Results: Among 77 DSD patients under this study, there were 43 (55.84%) 46, XX DSD cases, 30 (38.96%) 46, XY DSD cases, and 4 (5.19%) sex chromosome DSD cases. The age of presentation ranged from 0 days to 19 years with a mean of 2.29±4.2 years. Only 16.9% of the cases presented in their neonatal period. Almost all (98.7%) patients featured genital ambiguity. Congenital Adrenal Hyperplasia (CAH) had been found in all of the 46, XX DSD cases and in 1 sex chromosome DSD case. Among the patients with 46, XY DSD, 5 patients had PAIS (Partial Androgen Insensitivity Syndrome), 5 patients had CAIS (Complete Androgen Insensitivity Syndrome), 3 patients had 5αRD (5α Reductase Deficiency) and 5 patients had GD (Gonadal Dysgenesis). The gender of rearing was male in 25 (32.5%) cases and female in 52 (67.5%) cases. During the study period, 12 (15.58%) patients had undergone surgical intervention, 35 (45.54%) patients had been referred for surgery, 36(46.8%) patients were under hormonal therapy, and for 2 (2.6%) patient’s operation had been planned.
Conclusions: As according to the finding of this study, AIS was the most common etiological findings among 46, XY DSD cases, and CAH was exclusively present among all 46, XX DSD cases.
Downloads
References
Biason-Lauber A. Control of sex development. Best Practice & Research Clinical Endocrinology & Metabolism. 2010 Apr 1; 24(2):163–86.
Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015). Clin Endocrinol. 2016 May; 84(5):771–88.
Dar S, Nazir M, Lone R, Sameen D, Ahmad I, Wani W, et al. Clinical spectrum of disorders of sex development: A cross-sectional observational study. Indian J Endocrinol Metab. 2018; 22(6):774.
Lee PA, Houk CP, Ahmed SF, Hughes IA, International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006 Aug; 118(2): e488-500.
Hughes IA. Disorders of sex development: a new definition and classification. Best Pract Res Clin Endocrinol Metab. 2008 Feb; 22(1):119–34.
Warne GL, Zajac JD. Disorders of sexual differentiation. Endocrinol. Metab. Clin. North Am. 1998; 27: 945–67.
Maclean HE, Warne GL, Zajac JD. Intersex disorders: Shedding light on male sexual differentiation beyond SRY. Clin. Endocrinol. 1997; 46: 101–8.
Vainio SV, Heikkilä Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999; 397: 405–9.
Hamerton JL, Canning N, Ray M et al. A cytogenetic survey of 14069 newborn infants. Clin. Genet. 1975; 8: 223–43.
Dar SA, Nazir M, Lone R, Sameen D, Ahmad I, Wani WA, et al. Clinical spectrum of disorders of sex development: A cross-sectional observational study. Indian Journal of Endocrinology and Metabolism. 2018 Jan 11; 22(6):774.
Délot EC, Papp JC, Délot EC, Fox M, Grody W, Lee H, et al. Genetics of Disorders of Sex Development: The DSD-TRN Experience. Endocrinology and Metabolism Clinics of North America. 2017 Jun 1; 46(2):519–37.
Thyen U, Lanz K, Holterhus P-M, Hiort O. Epidemiology and Initial Management of Ambiguous Genitalia at Birth in Germany. HRP. 2006;66(4):195–203.
MacLaughlin DT, Donahoe PK: Sex determination and differentiation. N Engl J Med 2004; 350: 367–378.
Brennan J, Capel B: One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 2004; 5: 509–521.
Mortlock DP, Innis JW: Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet 1997; 15: 179–180.
Hiort O, Holterhus P: The molecular basis of male sexual differentiation. Eur J Endocrinol 2000; 142: 101–110.
Sax L: How common is intersex? A response to Anne Fausto-Sterling. J Sex Res 2002; 39: 174–178.
Pang SY, Wallace MA, Hofman L, Thuline HC, Dorche C, Lyon IC, Dobbins RH, Kling S, Fujieda K, Suwa S: Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 1988; 81: 866–874.
Merke DP, Bornstein SR: Congenital adrenal hyperplasia. Lancet 2005; 365: 2125–2136.
Dörr HG, Nennstiel-Ratzel U: AGS-Screening – Neugeborenenscreening auf das Adrenogenitale Syndrom mit 21-HydroxylaseDefekt. Kinderärztl Prax 2005; 76: 284–291
Riepe FG, Krone N, Viemann M, Partsch CJ, Sippell WG: Management of congenital adrenal hyperplasia: results of the ESPE questionnaire. Horm Res 2002; 58: 196–205.
Skakkebaek NE: Testicular dysgenesis syndrome. Horm Res 2003; 60(suppl 3):49.
Deeb A, Mason C, Lee YS, Hughes IA: Correlation between genotype, phenotype and sex of rearing in 111 patients with partial androgen insensitivity syndrome. Clin Endocrinol 2005; 63: 56–62.
Boehmer AL, Bruggenwirth H, van Assendelft C, Otten BJ, Verleun-Mooijman MC, Niermeijer MF, Brunner HG, Rouwe CW, Waelkens JJ, Oostdijk W, Kleijer WJ, van der Kwast TH, de Vroede MA, Drop SL: Genotype versus phenotype in families with androgen insensitivity syndrome. J Clin Endocrinol Metab 2001; 86: 4151–4160.
Boehmer AL, Brinkmann AO, Sandkuijl LA, Halley DJ, Niermeijer MF, Andersson S, de Jong FH, Kayserili H, de Vroede MA, Otten BJ, Rouwe CW, Mendonca BB, Rodrigues C, Bode HH, de Ruiter PE, Delemarre-van de Waal HA, Drop SL: 17Beta-hydroxysteroid dehydrogenase-3 deficiency: diagnosis, phenotypic variability, population genetics, and worldwide distribution of ancient and de novo mutations. J Clin Endocrinol Metab 1999; 84: 4713–4721
Kulkarni KP, Panigrahi I, Das R, Kaur S, Marwaha RK. Pediatric disorders of sex development. Indian J Pediatr. 2009 Nov 4; 76(9): 956.
Joshi RR, Rao S & Desai M. Etiology and clinical profile of female genitalia – an overview of 10 years of experience. Indian Paediatrics 2007 43 974–978.
Gupta DK, Menon PSN. Ambiguous genitalia—an Indian perspective. Indian J Pediatr. 1997 Mar; 64(2):189–94.
Nimkam S, Likitmoskul S, Sangecharoenkit P, Pathomvanich A, Sawathiparnich P, Wacharasindhu S, Punnakanta L, Angsusingha K & Tuchinda C. Ambiguous genitalia: an overview of 22 years’ experience
Al-Agha A, Thomsett M, Batch J. The child of uncertain sex: 17 years of experience. J Paediatr Child Health. 2001 Aug 31; 37(4):348–51.
Walia R, Singla M, Vaiphei K, Kumar S, Bhansali A. Disorders of sex development: a study of 194 cases. Endocrine Connections. 2018 Feb; 7(2):364–71.
Al-Jurayyan NAM. Ambiguous Genitalia, Two Decades of Experience: Clinical Management and Sex Assignment. Journal of Taibah University Medical Sciences. 2010 Jan 1; 5(1):13–20.
Gangaher A, Chauhan V, Jyotsna VP, Mehta M. Gender identity and gender of rearing in 46 XY disorders of sexual development. Indian J Endocrinol Metab. 2016; 20(4):536–41
Mota BC, Oliveira LMB, Lago R, Brito P, Canguçú-Campinho AK, Barroso U, et al. Clinical profile of 93 cases of 46, XY disorders of sexual development in a referral center. IntBraz J Urol off J BrazSoc Urol. 2015; 41(5):975–81.
Vasundhera C, Jyotsna VP, Kandasamy D, Gupta N. Clinical, hormonal and radiological profile of 46XY disorders of sexual development. Indian J EndocrinolMetab. 2016; 20(3):300–7.
Halder A. Disorder of sex development: Spectrum of disorders in a referral tertiary care hospital in North India. Global J Hum Genet Gene Ther. 2013; 1:77–89.
Maimoun L, Philibert P, Cammas B, Audran F, Bouchard P, Fenichel P, et al. Phenotypical, Biological, and Molecular Heterogeneity of 5α-Reductase Deficiency: An Extensive International Experience of 55 Patients. The Journal of Clinical Endocrinology & Metabolism. 2011 Feb; 96(2):296–307.
Gupta DK, Menon PSN. Ambiguous genitalia: An Indian perspective. Indian J Pediatr. 1997; 64:189–94. [PubMed: 10771835]