Current mutation-targeted DMD treatments and their theoretical application in a sub-group of Albanian patients
Abstract
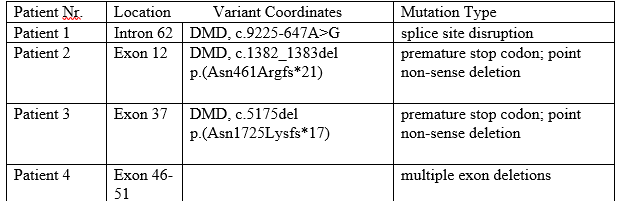
Although the molecular origins of Duchenne muscular dystrophy have been known for several years, there is still no curative treatment for the disease. Exon skipping is a mutation-specific approach; which exon to skip depends on the size and location of the mutation. As such, having a genetic diagnosis of the disease is important. The genetic diagnosis mentioned in this paper was made privately in a sub-group of DMD patients from our clinic. Nine out of fourteen patients (64%) have mutations that are targeted by therapies that are currently licensed or undergoing Phase III trials.
Downloads
References
Emery, A. E. (2002). The muscular dystrophies. The Lancet, 359(9307), 687-695. doi:10.1016/s0140-6736(02)07815-7
Worton, R. G., & Thompson, M. W. (1988). Genetics of Duchenne Muscular Dystrophy. Annual Review of Genetics, 22(1), 601-629. doi:10.1146/annurev.ge.22.120188.003125
Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919-28. DOI: 10.1016/0092-8674(87)90579-4. PMID: 3319190.
Cohn, R. D., & Campbell, K. P. (2000). Molecular basis of muscular dystrophies. Muscle & Nerve, 23(10), 1456-1471. doi:10.1002/1097-4598(200010)23:103.0.co;2-t
Beytía Mde L, Vry J, Kirschner J. Drug treatment of Duchenne muscular dystrophy: available evidence and perspectives. Acta Myol. 2012;31(1):4-8.
Manzur AY, Kinali M, Muntoni F. Update on the management of Duchenne muscular dystrophy. Arch Dis Child. 2008 Nov;93(11):986-90. DOI: 10.1136/adc.2007.118141. Epub 2008 Jul 30. PMID: 18667451.
Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2(12):731-740. doi:10.1016/s1474-4422(03)00585-4
Pichavant C, Aartsma-Rus A, Clemens PR, et al. Current status of pharmaceutical and genetic therapeutic approaches to treat DMD. Mol Ther. 2011;19(5):830-840. doi:10.1038/mt.2011.59
Erik H. Niks & Annemieke Aartsma-Rus (2017) Exon skipping: a first in class strategy for Duchenne muscular dystrophy, Expert Opinion on Biological Therapy, 17:2, 225-236, DOI: 10.1080/14712598.2017.1271872
Aartsma-Rus A, Fokkema I, Verschuuren J, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Human Mutation. 2009 Mar;30(3):293-299. DOI: 10.1002/humu.20918.
Bushby K, Finkel R, Wong B, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014;50(4):477-487. doi:10.1002/mus.24332
Deconinck, N., & Dan, B. (2007). Pathophysiology of Duchenne Muscular Dystrophy: Current Hypotheses. Pediatric Neurology, 36(1), 1-7. doi:10.1016/j.pediatrneurol.2006.09.016