Effect of misoprostol versus oxytocin in reducing postpartum hemorrhage after labor induction
Abstract
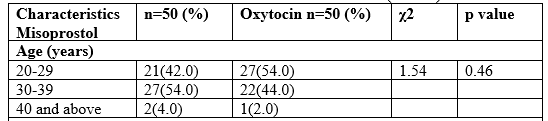
Introduction: Postpartum hemorrhage (PPH) is a life-threatening obstetric emergency that occurs after caesarean section (CS) or normal vaginal delivery (NVD). It may be defined as ≥500 mL hemorrhage after vaginal or ≥1000 mL hemorrhage after CS delivery. PPH is one of the most common obstetric maternal complications and is among the three most common etiologies of maternal death worldwide. Objective: To compare low dose sublingual misoprostol with the standard 10 IU of intramuscular oxytocin in active management of third stage of labor. Materials and Methods: The study was a randomized clinical trial carried out at the Department of Gynaecology & Obstetrics, 250 Bedded General Hospital, Noakhali, Bangladesh from July to December 2021. One hundred (100) patients were included. Women with term pregnancy were randomized to receive either 200 µg misoprostol sublingually or 10 IU oxytocin intramuscularly after vaginal delivery. Primary outcome measured was mean blood loss and incidence of primary postpartum hemorrhage (PPH). Secondary outcome measured included duration of third stage of labor, side effects of drugs and need for additional oxytocics to treat life‑threatening hemorrhage. Results: Total 100 women with term pregnancy in two groups of 50 each were studied. The mean blood loss with sublingual misoprostol and oxytocin groups was 320.58 ± 244.12 vs. 253.27 ± 171.74 ml; (P = 0.11). The mean duration of third stage of labor was similar and the difference was not statistically significant (6.65 ± 3.47 vs. 6.08 ± 3.07 minutes) (P = 0.38), as well as need for additional oxytocics (14.0% vs. 6.0% P = 0.18) misoprostol and oxytocin, respectively. There were no differences at the 5% level of significance between groups with regard to the incidence of PPH (20.0% vs. 14.0% respectively; P=0.43). Among the women who were recruited (safety population), the frequencies of the expected side effects did not differ significantly between the two groups. In misoprostol group, side effects were shivering, fever, nausea and abdominal pains, while the oxytocin group abdominal pains, headaches and shivering. Conclusion: Misoprostol administered in the third stage of labor after labor induction by Oxytocin showed a trend towards significantly reducing postpartum blood loss and incidence of postpartum hemorrhage. Sublingual misoprostol has similar efficacy to standard intramuscular oxytocin in preventing PPH following vaginal birth. Misoprostol at 200 µg with its thermostability may be an effective alternative to intramuscular oxytocin in active management of third stage of labor.
Downloads
References
Andolina K, Daly S, Roberts N, et al. Objective measurement of blood loss at delivery: is it more than a guess? American Journal of Obstetrics & Gynecology. 1999;180:p. S69.
Ueland K. Maternal cardiovascular dynamics. VII. Intrapartum blood volume changes. American Journal of Obstetrics and Gynecology. 1976; 126(6) :671–677.
Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. American Journal of Obstetrics and Gynecology. 2008;199(5):p. 519.
Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews. 2007;(1)CD003249
Lu MC, Fridman M, Korst LM, et al. Variations in the incidence of postpartum hemorrhage across hospitals in California. Maternal and Child Health Journal. 2005;9(3):297–306.
Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. American Journal of Obstetrics and Gynecology. 2010;202(4):p. 353.
Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011– 2013. Obstet Gynecol. 2017; 130:366-73.
Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2:e323-33.
WHO, UNICEF, UN Population Fund and the World Bank. Trendsin maternal mortality: 1990 to 2010. WHO, UNICEF, UNFPA and The World Bank estimates. Geneva: World Health Organization, 2012.
Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009; 31:980-93.
Atukunda EC, Mugyenyi GR, Obua C, et al. Measuring postpartum haemorrhage in low resource settings: the diagnostic validity of weighed blood loss versus quantitative changes in hemoglobin. PloS One 2016; 11:e0152408.
Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2014; 2:CD003249.
Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large nationwide sample of deliveries. Anesth Analg. 2010; 110:1368-1373.
Wetta LA, Szychowski GM, Seals S, et al. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol. 2013; 209:51- e1.
Begley CM, Gyte GM, Devane D, Mcguire W, Weeks A. Active versus management for women in the third stage of labour. Cochrane Database Syst Rev. 2015; 3:CD007412.
Jangsten E, Mattsson L, Lyckestam I, Hellstorm A, Berg A. A comparison of active management and expectant management of the third stage of labour: a Swedish randomized controlled trial. BJOG. 2011; 118:362-369.
Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014; 6:CD00133.
Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Med J 2010; 51:207-11.
Oboro VO, Tabowei TO. A randomized controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. J. Obstet Gynaecol 2003; 23:13-6.
Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramusanlar oxytocin for prevention of post-partum haemorrhage in low-risk women. Int J Gynaecol Obstet 2012; 116:138-42.
Patel A, Goudar SS, Geller SE. Drape estimation versus visual assessment. Int J Gynaecol Obstet 2006; 93:320-4.
Enakpene CA, Morhason-Bello IO, Enakpene CO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post-partum hemorrhage during third stage of labour. J Obstet Gynaecol Res 2007;33:810-7.
Badejoko OO, Ijarotimi OI, Awowole IO, Loto OM, Badejoko BO, Olaiya D, et al. Adjunctive rectal misoprostol versus oxytocin infusion for prevention of post-partum haemorrhage in women at risk: A randomized controlled trial. J Obtet Gynaecol Res 2012; 38:1294-301.
Robinson C, Schumann R, Zhang P, Young RC. Oxytocin induced desensitization of the oxytocin receptor. Am J Obstet Gynecol. 2003; 188:497-502.