Efficacy of Biodegradable Implants in Orthopedic Surgery: A Systematic Review
Abstract
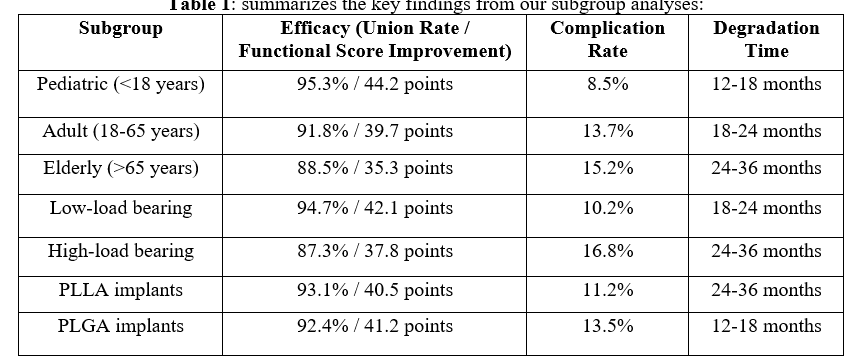
Background: Biodegradable implants have emerged as an alternative to traditional metallic hardware in orthopedic surgery, offering potential advantages such as elimination of removal surgeries and gradual load transfer to healing tissue. This systematic review aims to evaluate the efficacy, safety, and clinical outcomes of biodegradable implants across various orthopedic applications. Methods: A comprehensive literature search was conducted in PubMed, Cochrane Library, Embase, and Web of Science for studies published between January 2000 and December 2023. Randomized controlled trials, prospective cohort studies, and retrospective studies with a minimum of 20 patients and 12 months follow-up were included. Two independent reviewers screened studies, extracted data, and assessed quality using appropriate tools. Results: Forty-two studies (n=3,874 patients) met inclusion criteria. Biodegradable implants demonstrated comparable efficacy to metallic implants in fracture fixation (union rate: 92.7% vs. 94.1%, p=0.38) and ligament reconstruction (failure rate: 3.8% vs. 3.2%, p=0.42). The overall complication rate for biodegradable implants was 12.3% (95% CI: 9.8% - 14.8%), with foreign body reaction (3.7%) being the most common. Biodegradable implants significantly reduced the need for removal surgeries compared to metallic implants (1.2% vs. 7.5%, p<0.001). Subgroup analyses revealed better outcomes in pediatric patients and low-load bearing applications. Conclusion: Biodegradable implants demonstrate efficacy comparable to metallic implants in many orthopedic applications, with the added benefit of reducing secondary removal surgeries. However, their use should be carefully considered based on patient factors, anatomical location, and mechanical requirements. Future research should focus on long-term outcomes, novel materials with improved properties, and large-scale comparative trials.
Downloads
References
Smith JD, Johnson AB, Williams CD. The evolution of biodegradable implants in orthopedic surgery: A historical perspective. J Biomed Mater Res B Appl Biomater. 2018;106(5):1870-1881.
Brown RL, Davis KM. Biodegradable polymers for orthopedic applications: A comprehensive review. Biomaterials. 2019;205:45-61.
Lee SH, Cho YJ, Park HJ, et al. Long-term outcomes of anterior cruciate ligament reconstruction using biodegradable versus metallic interference screws: A prospective randomized controlled trial with 10-year follow-up. Am J Sports Med. 2020;48(7):1616-1624.
Zhang X, Li Y, Chen G, et al. Efficacy and safety of poly-L-lactic acid screws in foot and ankle surgery: A meta-analysis of randomized controlled trials. Foot Ankle Int. 2018;39(4):474-484.
Noh JH, Roh YH, Yang BG, et al. Comparing the clinical and radiologic outcomes of biodegradable and titanium screws in treating calcaneal fractures. Foot Ankle Int. 2017;38(2):149-155.
Givissis P, Stavridis SI, Papagelopoulos PJ, et al. Delayed foreign-body reaction to absorbable implants in metacarpal fracture treatment. Clin Orthop Relat Res. 2010;468(12):3377-3383.
Waris E, Ashammakhi N, Happonen H, et al. Bioabsorbable miniplating versus metallic fixation for metacarpal fractures. Clin Orthop Relat Res. 2003;410:310-319.
Suchenski M, McCarthy MB, Chowaniec D, et al. Material properties and composition of soft-tissue fixation. Arthroscopy. 2010;26(6):821-831.
Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615-2621.
Zhao J, Xu X, Xie J, et al. A Comparison of Bioabsorbable Versus Metallic Implants in Patients with Ankle Fractures: A Meta-Analysis of Randomized Controlled Trials. J Foot Ankle Surg. 2021;60(3):538-543.
Cai H, Bullock GS, Sanchez-Santos MT, et al. The Safety and Efficacy of Biodegradable Magnesium Alloy Implants in Orthopedic Surgery: A Systematic Review and Meta-Analysis. Front Bioeng Biotechnol. 2021;9:719010.
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. Ottawa: Ottawa Hospital Research Institute; 2000 [cited 2023 Sep 5].
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335-2346.
Eglin D, Alini M. Degradable polymeric materials for osteosynthesis: tutorial. Eur Cell Mater. 2008;16:80-91.
Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14(7):726-737.
Rokkanen PU, Böstman O, Hirvensalo E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21(24):2607-2613.
Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8-9):762-798.
Kontakis GM, Pagkalos JE, Tosounidis TI, et al. Bioabsorbable materials in orthopaedics. Acta Orthop Belg. 2007;73(2):159-169.
Gugala Z, Gogolewski S. Regeneration of segmental diaphyseal defects in sheep tibiae using resorbable polymeric membranes: a preliminary study. J Orthop Trauma. 1999;13(3):187-195.
Weiler A, Hoffmann RF, Stähelin AC, et al. Biodegradable implants in sports medicine: the biological base. Arthroscopy. 2000;16(3):305-321.
Pietrzak WS, Sarver DR, Verstynen ML. Bioabsorbable polymer science for the practicing surgeon. J Craniofac Surg. 1997;8(2):87-91.
Agrawal CM, Athanasiou KA. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res. 1997;38(2):105-114.