Rate and Pattern of Drug Reaction among Tuberculosis Patient During Antitubercular Drug Therapy
Abstract
Background: Tuberculosis (TB) treatment often involves multi-drug regimens that can lead to adverse drug reactions (ADRs). These ADRs may affect treatment adherence and outcomes. In the treatment of tuberculosis (TB), the use of multi-drug regimens has been linked to unfavorable adverse drug reactions (ADRs). This study aims to evaluate the rate of ADRs and their effects on TB treatment at National Institute of Diseases of the Chest and Hospital with a focus on patients with risk factors.
Objective: To assess the rate and pattern of adverse drug reactions (ADRs) caused by anti-TB medications in the infectious disease department throughout 12 months. To identify known adverse drug reactions (ADRs) that are severe and avoidable.
Methods: A retrospective study was conducted to evaluate the rate and pattern of drug reactions in tuberculosis patients receiving antitubercular therapy. This cross-sectional study was conducted via retrospective review of outpatients’ medical records. Details regarding ADRs were identified by a pharmacist and verified by a consultant respiratory physician. Data were analyzed by SPSS software (version 25.0, IBM statistical product).
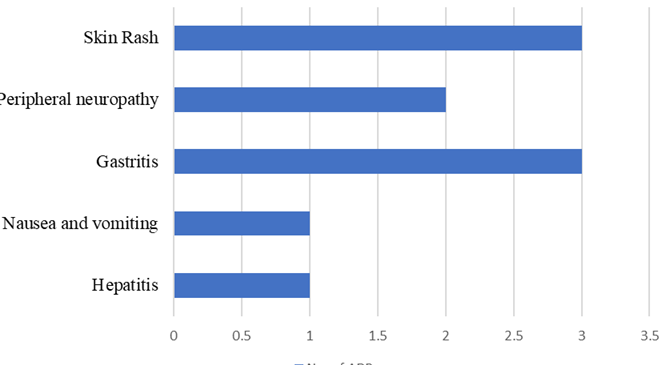
Results: Of the 50 patients, 88% were male with a mean age of 47.8 ± 14.18 years. Twenty percent of patients experienced ADRs, with gastrointestinal issues and skin rashes being the most common. Seventy percent of ADRs occurred within 3 weeks of starting the intensive phase of treatment. Diabetes mellitus was the most prevalent comorbidity (60% of patients). While not statistically significant, trends suggested higher ADR rates in smokers and long-term diabetics. All ADRs were managed symptomatically without discontinuing antitubercular therapy.
Conclusion: The ADR rate in this study population was comparable to that reported in patients without comorbidities. Gastrointestinal ADRs were most common, and most ADRs occurred early in treatment. While comorbidities didn't significantly increase ADR risk, the high prevalence of diabetes underscores the need for integrated TB-diabetes care. Close monitoring for ADRs, especially during the initial weeks of treatment and in patients with risk factors, is crucial for successful TB management.
Downloads
References
Tuberculosis 2023. https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
Tuberculosis SEARO 2019. https://www.who.int/bangladesh/health-topics/tuberculosis.
B J, Antony L, V B, A D. Pattern of adverse drug reactions of antitubercular drugs in tuberculosis patients with comorbidities and risk factors in South Indian government health-care facilities. National Journal of Physiology Pharmacy and Pharmacology 2021:1. https://doi.org/10.5455/njppp.2021.11.07220202105072021.
Sinha K, Marak IT, Singh W. Adverse drug reactions in tuberculosis patients due to directly observed treatment strategy therapy; experience at an outpatient clinic of a teaching hospital in the city of Imphal, Manipur, India. J Assoc Chest Physicians 2013; 1:50.
Dedun A, Borisagar G, Solanki R. Impact of adverse drug reaction of first line anti-tuberculous drugs on treatment outcome of tuberculosis under revised national tuberculosis control programme. Int J Adv Med 2017; 4:645.
Singh A, Prasad R, Balasubramanian V, Gupta N, Gupta P. Prevalence of adverse drug reaction with first-line drugs among patients treated for pulmonary tuberculosis. Clin Epidemiol Glob Health 2015;3: S80-90.
Ma Y, Che NY, Liu YH, Shu W, Du J, Xie SH, et al. The joint impact of smoking plus alcohol drinking on treatment of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 2019; 38:651-7.
Pant R, Pandey K, Joshi M, Sharma S, Pandey T, Pandey S. Risk factor assessment of multidrug-resistant tuberculosis. J Nepal Health Res Council 1970; 7:89-92.
Athira B, Manju CS, Jyothi E. A study on adverse drug reactions to first line anti-tubercular drugs in DOTS therapy. Int J Pharmacol Clin Sci 2015; 4:7-11.
Dhingra VK, Rajpal S, Aggarwal N, Aggarwal JK, Shadab K, Jain SK. Adverse drug reactions observed during DOTS. J Commun Dis 2004; 36:251-9.
Gholami K, Kamali E, Hajiabdolbagh M, Shalviri G. Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients. Pharm Pract (Granada) 2006; 4:134-8.
Sivaraj R, Umarani S, Muralidhar P, Parasuraman S. Revised national tuberculosis control program regimens with and without directly observed treatment, short-course: A comparative study of therapeutic cure rate and adverse reactions. Perspect Clin Res 2014; 5:16-9.
Sadiq S, Gupta S, Khajuria V, Tandon VR, Mahajan A, Gupta M. Adverse drug events due to antiretroviral therapy in a Northern Indian tertiary care institution. Natl J Physiol Pharm Pharmacol 2016; 6:205-8.